- validation of the GAMP5 computer system,
- FDA 21CFR11 requirements for e-signatures and e-records,
- process modelling based on ISA S88.01.
Pharma
Automation system
Tools
We use established universal tools:
- PLC level: Siemens, others upon request,
- SCADA: GE iFix, Siemens WinCC, others upon request,
- production informatics level: MS SQL and Reporting server, MS .NET, GE Historian,
- with the open concept we are able to integrate the solution into the customer’s environment.
The automation system ensures
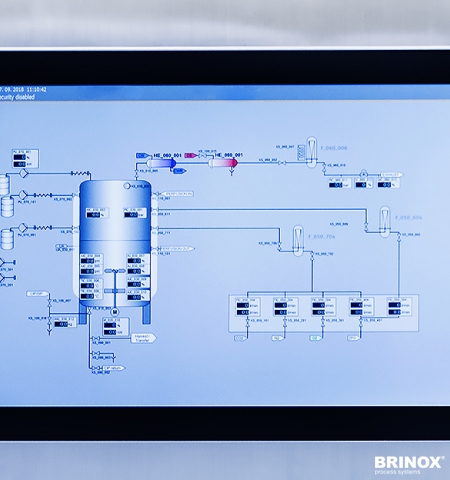
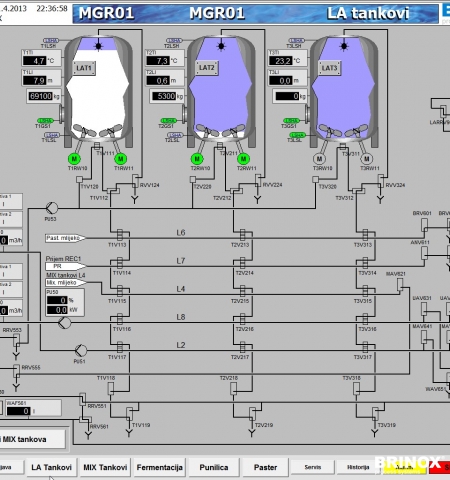
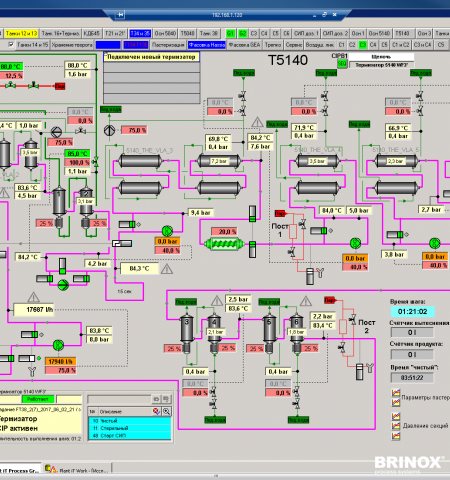
- The advanced SCADA visualisation allows to control all elements of a process (valves/motors, sensors).
- The use of the ISA S88.01 recipe system ensures a high level of automation and at the same time makes the process highly adaptable and allows good use of the process equipment.
- The modular hierarchic control system concept ensures the execution of the process in various procedure levels (stages, operations, unit procedures, procedures) and consequently the process optimisation and a faster introduction of new products.
- The user independently creates recipes based on the provided templates which protects their know-how and product.
- The recipe approval work process is based on e-signatures.
- The unit procedures run entirely on the PLC-level which ensures reliable operation and fast changeovers between individual steps.
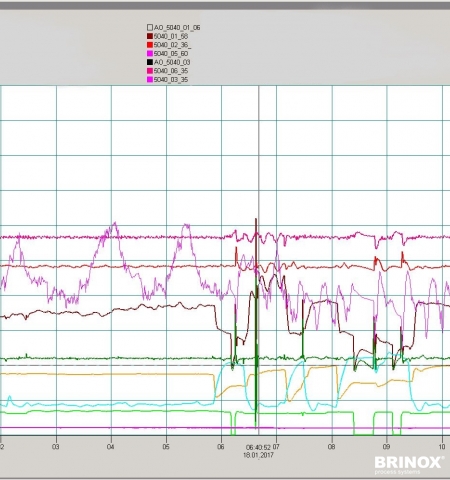
- The recipe and process execution data, historic data and the Audit Trail provide an extensive database for electronic reporting.
- The FDA 21CFR11 support is based on Windows protection.
- The implementation of interfaces to other systems at the PLC level (supply of clean media, safety systems, etc.) and at the information level (MES, KPI).